Micellar water, a product found in supermarkets, chemists, and bathroom cabinets around the world, is commonly used to remove makeup. It’s a very effective cleanser, and many people swear by it as part of their skincare routine.
So what is micellar water, and why is it so good at getting makeup and sunscreen off? Here’s the science.
What are micelles?
Oil and water generally don’t mix, which is why you’ll struggle to remove makeup and sunscreen (which both contain oils) with just plain water.
But micellar water products contain something called micelles — clusters of molecules that are very effective at removing oily substances. To understand why, you must first know two chemistry terms: hydrophilic and hydrophobic.
A hydrophilic substance “loves” water and mixes easily with it. Salt and sugar are examples.
A hydrophobic substance “hates” water and generally refuses to mix with it. Examples include oil and wax.
Hydrophilic materials will happily mix with other hydrophilic materials. The same goes for hydrophobic substances. But if you try to combine hydrophilic and hydrophobic materials, they won’t mix.
How are micelles formed? It’s all about surfactants
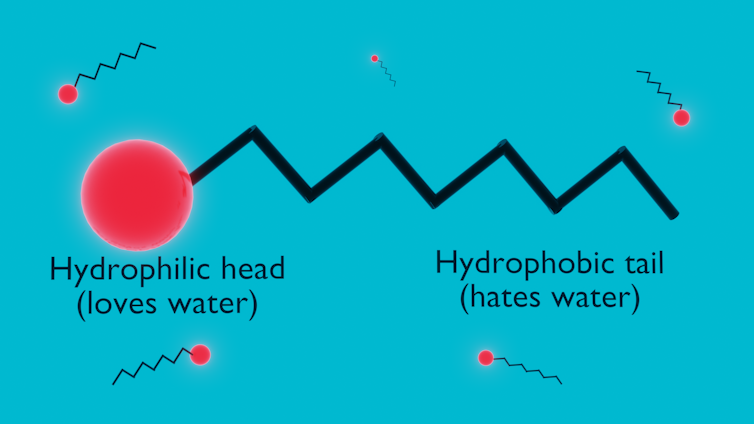
The micelles in micellar water are formed by special molecules known as surfactants.
Surfactant stands for surface active agent. These molecules looked at their hydrophilic and hydrophobic brethren and said, why not both? They are typically comprised of two ends: a head group that is hydrophilic and a hydrophobic tail.
When a small amount of surfactant is added to water, the two ends of the molecule have competing interests. The hydrophilic head wants to be in the water, but the hydrophobic tail can’t stand water.
Add enough surfactant, and eventually, we will pass a critical micelle concentration, and the surfactants will self-assemble into clusters of approximately 20 to 100 surfactant molecules.
All the hydrophilic heads will be pointing outward, while the hydrophobic tails remain “hidden” at the center. These clusters are micelles.
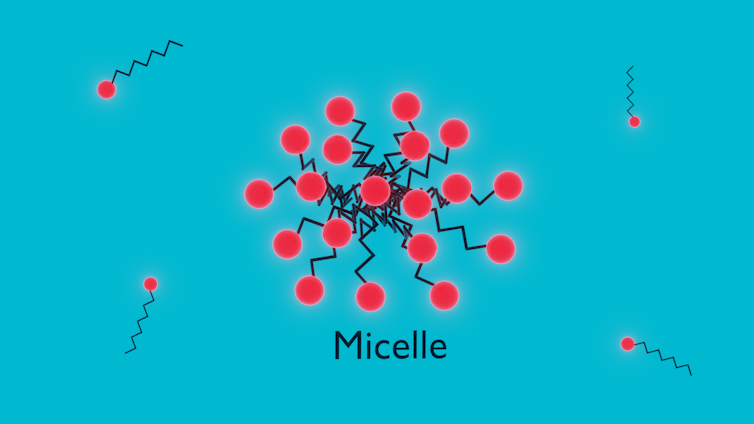
These micelles have a hydrophilic exterior, meaning they are happy to remain mixed throughout the water. However, in the center remains a hydrophobic pocket that’s very good at attracting oils.
This is very handy and helps explain why adding some detergent (a surfactant) to water will allow you to wash an oily saucepan. The surfactant first helps lift the oil, and then the oil can remain mixed into the water, finding a new home in the hydrophobic center of the micelle.
Micellar water in action
Surfactants are in your dishwashing detergent, body wash, shampoo, toothpaste, and even many foods. In all these cases, they help the water interact with the dirt and oils, and micellar water is no different.
When you apply some micellar water to a cotton pad, another convenient interaction occurs. Wet cotton is hydrophilic (loves water). Consequently, some of the micelles will unravel, with the hydrophilic heads being attracted to the damp cotton pad.
Now, sticking out from the surface will be a layer of hydrophobic tail groups. These hydrophobic tails cannot wait to attract themselves to makeup, sunscreen, oils, dirt, grease, and other contaminants on your face.
As you sweep the cotton pad across your skin, these contaminants bind to the hydrophobic tails and are removed from the skin.
Some contaminants will also find themselves encapsulated in the hydrophobic centers of the micelle.
Either way, a cleaner surface is left behind.
Look at how a cotton wipe soaked in micellar water cleans up a small oil spill compared to water alone.
So why shouldn’t I just use dishwashing detergent to wash my face?
Technically, that would work, as detergent contains lots of micelle-forming surfactants.
But these particular surfactants would probably cause a lot of skin and eye irritation while also damaging and drying out your skin. Not nice.
The surfactants in micellar water are chosen to be mild and well tolerated by most people’s skin. But micellar water isn’t the only skincare product to contain micelles. There are many other face-cleaning products that also make great use of surfactant molecules and work very well.
Now, it’s not perfect. While it is effective at removing a wide range of contaminants, thick or heavy makeup might not come off easily with micellar water (you might need to do a more vigorous clean).
Some products say there is “zero residue”, although the fine print clearly states this refers to visible residue.
Many products also state there is no rinse-off required. Surfactants will remain on your skin after product use, but they don’t cause irritation for most people. If your skin is feeling irritated after using a micellar water product, you can try rinsing afterward or discontinuing use.
As is the case with many cosmetic products, you should test it first on a small patch of skin before using it all over your face.
Daniel Eldridge, Senior Lecturer in Chemistry, Swinburne University of Technology
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Follow us on X, Facebook, or Pinterest